regulation
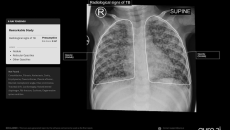
Its chest X-ray analysis AI tool is the first to receive Europe's clearance for use in children aged 0-3.
Industry regulatory leaders outline the best approaches for digital health companies looking to make sense of FDA's long-in-the-tooth enforcement discretion guidelines.
The regulator imposed a series of requirements for the tech companies that must be followed for at least 10 years.
An annual review of state and federal laws regarding behavioral or mental health telemedicine highlights increased interest in the modality from policymakers and regulators.
More expertise will be necessary if the regulator is to keep up with the constant evolution of real-world evidence, privacy and other data-driven challenges, Dr. Hahn said.
Bradley Merrill Thompson, an FDA-watcher, lawyer and occasional MobiHealthNews guest contributor, joins the MobiHealthNews team for a closer look at what the FDA has been up to around digital health and AI.
A new guidance from the U.S. regulator relaxes a handful of requirements for these digital health devices, and clarifies the agency's long-term enforcement policy for low-risk wellness products.
Dr. Yuri Maricich, chief medical officer at Pear Therapeutics, speaks on his company's experience bringing a digital therapeutic product through the regulator's experimental program for health software.
Nurx, Carbon Health and Everlywell have officially halted their mail-order testing-kit services. But at least one startup is still moving to secure an Emergency Use Authorization for its at-home rapid serology diagnostic.
Digital health innovation consultant Naomi Fried highlights trends to watch among pharmas interested in digital health technologies.