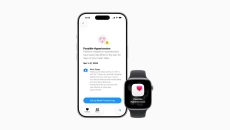
FDA 510(k) clearance
The feature uses machine learning and heart sensor data to flag signs of hypertension and will roll out to Watch Series 9, 10 and 11 as well as Ultra 2 and 3 by month’s end.
The company has secured 510(k) clearance for its Apple Watch-powered seizure monitoring platform, expanding the role of wearables in epilepsy care.
The company's product, Prism, uses neurofeedback to help PTSD patients learn to control their emotional response.
This marks the fourth clearance for Volpara’s breast health platform since its original authorization back in 2010.
The system is designated to be used by clinicians to monitor patients at home or in the healthcare setting.
The INVU pregnancy monitoring system offers an alternative to existing methods of measuring uterine activity.
The company has developed a line of products targeting the mammography space to enable early and accurate breast cancer detection.
The system was cleared for use among individuals 12 years old and up with Type 1 or Type 2 diabetes on multiple-dose injection therapy.
Nerivio is a wireless, noninvasive remote electrical-stimulation wearable that is worn on the upper arm at the onset of a migraine.
Using this tool can help radiologists speed up the diagnosis process and reduce missed cases, according to the company.